|
Neuronal
differentiation and its physiological and pharmacological modulation: role of
the trancription factor REST Nervous system
development relies on a complex signalling network to engineer the orderly
transitions that lead to the acquisition of a neural cell fate (Ballas and
Mandel, 2005). Progression from the non neuronal pluripotent stem cell to a
restricted neural lineage is characterized by distinct patterns of gene
expression, particularly the restriction of neuronal gene expression to neurons
(Ballas and Mandel, 2005). The Repressor
Element 1 Silencing Transcription Factor (REST), also known as the
Neuron-restrictive Silencer Factor (NRSF), plays a pivotal role in such a
context as it maintains transcriptional silencing of a range of neuronal genes
in differentiated non-neuronal cells, as well as in un-differentiated neuronal
cells during early lineage commitment in neurogenesis. REST, in fact,
was originally discovered as a transcriptional repressor of a large number of
primarily terminal neuronal differentiation genes in non-neuronal cells and
neuronal stem cells (NSCs); its transcription is generally blocked as NSCs
undergo differentiation (Majumder, 2006), so that neuronal development can
occur properly. However, REST is expressed in some differentiated neurons and, when bound to a double-stranded small RNA, it is able to function also as an activator of its target gene transcription (Majumder et al., 2006), thus suggesting for REST a complex regulatory function in both embryonic and adult neuronal differentiation.
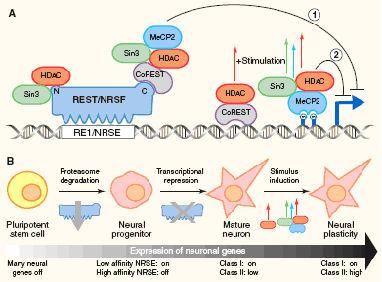
REST contains a
DNA-binding domain and two distinct repressor domains, which can interact with
several cellular repressor complexes, including huntintin protein (Htt) in the
cytoplasm and mSin3A, histone deacetylases (HDACs), N-Cor, CoREST, histone
H3-K9 methyltransferase G9a (HMTase), histone H3-K4 demethylase LSD1 (HDMase),
DNA methyl transferase 1 (DNMT1), DNA-methyl-CpG-binding protein-2 (MeCP2) and
chromatin remodelling complexes SWI/SNF in the nucleus (Fig. 1.1). Therefore REST serves as a giant
communication hub for the cell, having a variety of roles in normal development
as well as causing several abnormalities when deregulated (Majumder, 2006). We are
investigating how neuronal differentiation is determined in human and rat
cellular systems: particularly, we are studying IGF-I-, phorbol esthers- and
retinoids-induced differentiation of SH-SY5Y human neuroblastoma cells and
H-19/7 rat hippocampal neurons. Our goal is to unravel cellular and molecular
processes responsible for proper neuronal fate acquisition as well as for
aberrant differentiation occurring in tumors. Normal
development and abnormal development are, in fact, two sides of the same coin.
|
. |