|
Opioid receptorsí (MOP, NOP, s1)
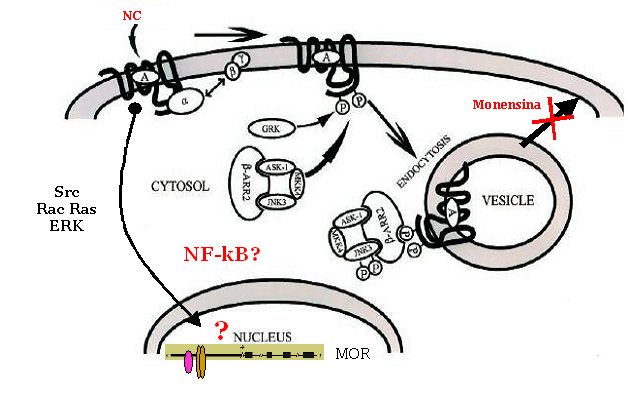
signalling and trafficking Internalization,
recycling and signalling of the human Mu-opioid receptor (MOPr) and of the
nociceptin receptor (hNOP) Prolonged
activation of G protein-coupled receptors (GPCRs) leads to a greatly decreased
sensitivity of the receptor to a subsequent agonist challenge. The molecular
mechanisms that lead to agonist-dependent desensitization of GPCRs are not
fully defined but several distinct events are involved, including uncoupling of
receptors from their heterotrimeric G proteins and reduction in the number of
receptors at the cell surface by either internalization or down-regulation
which may be associated with degradation. Furthermore,
receptor activation triggers signal transduction pathways that induce/repress
transcription factorsí activity, thus altering the cellular gene expression
profile. Moreover, opioid receptor may form omo-and hetero-dimers as well as they may interact with other receptor classes (e.g., tyrosine kinase receptors).
Therefore, we are
investigating MOPr internalisation and trafficking mediated by endomorphin-1
analogs in SH-SY5Y cells and in HEK-293 cells expressing MOPr. We are also
developing tagged MOPr in order to investigate receptor internalisation and
recycling by immunohistochemistry and confocal microscopy. Similarly, we are
investigating NC-promoted internalization of the human NOP (hNOP) receptor
occurring in the neuroblastoma cell line SK-N-BE or of the cloned human
receptors expressed in CHO cells by measuring the loss of binding sites for the
hydrophilic ligand [3 H]-NC in viable cells and by confocal microscopy
analysis. These events are related to desensitization of this receptor in
intact cells by investigating different signalling pathways activated by this
receptor. Recently we found out that NOPr and IGF-Ir signalling pathways converge at ERKs level: therefore, we are trying to unravel cellular and molecular processes involved in such cross-talk and to understand its biological meening. Characterization of novel sigma ligands and role of sigma1 receptors in the regulation of autonomic functions. Sigma (s) recognition sites are a unique class of binding sites, distributed in the nervous system and in peripheral organs acting as receptors for some unidentified endogenous ligand.They bind an array of structural classes of compounds including haloperidol and (+)-benzomorphans, such as (+)-pentazocine. We are investigating their role in the regulation of autonomic functions since they may interfere with neurotransmitter release, modulating their action on innervated tissue. We found distinguishable populations of sites in the rabbit iris-ciliary body and evaluating their contribution in the regulation of intraocular pressure in the rabbit. In vivo findings are completed by in vitro analysis of their role on electrically stimulated release of [3 H]NE from postganglionic sympathetic neurons and on isoproterenol-induced cAMP accumulation in isolated iris-ciliary body. These findings may prove a contribution of sigma ligands in the control of ocular hypertension.
|