|
Interactions
between a transcription factor and its target genes clearly require the
transcription factor to be expressed in the right place at the right time and
the presence of the corresponding recognition elements in the target gene. Just
as importantly, we need to know the molecular interactions that direct the
actions of these activators and repressors to specific promoters. We are
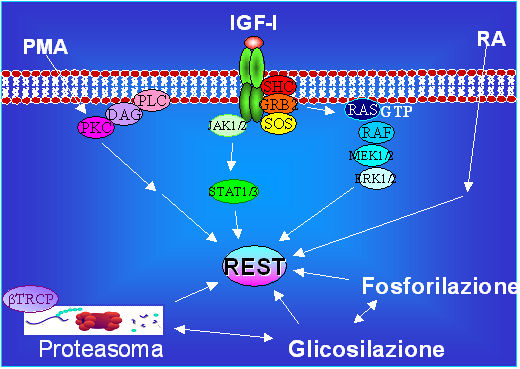
particularly interested in examining the role of REST/NRSF during neuronal
differentiation. The original proposed role for REST was that of a factor
responsible for restricting neuronal gene expression to the nervous system by
silencing expression of these genes in non-neuronal cells. Although it was
initially thought to repress neuronal genes in non-neuronal cells, the role of
REST is complex and tissue dependent. Moreover, it should be considered that
REST may function in different ways at different stages of cell differentiation
as a result of ch Finally, the glycosylation pattern of the REST protein
is analysed, moving from the observation that the molecular weight calculated
on REST sequence is about 116 kDa but using western blotting this transcription
factor appears to have distinct apparent molecular weight: this difference
could be explained by post-translational modifications of the proteins, like
glycosylation. In fact recently, several studies underlined the importance of
O-glycosylation in modulating transcriptional silencing, protein
phosphorylation, protein degradation by proteasome and protein–protein
interactions. |